How Does An Epigenetic Change Differ From A Mutation
The behavior of a person's genes doesn't just depend on the genes' DNA sequence - it'southward besides affected by so-called epigenetic factors. Changes in these factors tin can play a critical role in illness.
The external surround's furnishings upon genes can influence disease, and some of these effects can be inherited in humans. Studies investigating how environmental factors affect the genetics of an private's offspring are difficult to design. However, in certain parts of the globe in which social systems are highly centralized, ecology information that might have influenced families can exist obtained. For example, Swedish scientists recently conducted investigations examining whether diet afflicted the death rate associated with cardiovascular illness and diabetes and whether these effects were passed from parents to their children and grandchildren (Kaati et al., 2002). These researchers estimated how much access individuals had to food by examining records of annual harvests and food prices in Sweden across 3 generations of families, starting as far back equally the 1890s. These researchers plant that if a father did non have enough food available to him during a disquisitional period in his development simply before puberty, his sons were less likely to dice from cardiovascular affliction. Remarkably, death related to diabetes increased for children if nutrient was plentiful during this critical period for the paternal grandfather, only information technology decreased when excess food was available to the father. These findings suggest that diet can crusade changes to genes that are passed downward though generations by the males in a family unit, and that these alterations can affect susceptibility to certain diseases. Only what are these changes, and how are they remembered? The answers to questions such as these lie in the concept of epigenetics.
What Is Epigenetics? How Do Epigenetic Changes Affect Genes?
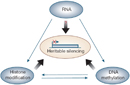
Epigenetics involves genetic control by factors other than an individual's Dna sequence. Epigenetic changes can switch genes on or off and determine which proteins are transcribed.
Epigenetics is involved in many normal cellular processes. Consider the fact that our cells all accept the same DNA, but our bodies incorporate many dissimilar types of cells: neurons, liver cells, pancreatic cells, inflammatory cells, and others. How tin can this be? In short, cells, tissues, and organs differ considering they have certain sets of genes that are "turned on" or expressed, as well equally other sets that are "turned off" or inhibited. Epigenetic silencing is ane way to turn genes off, and information technology tin contribute to differential expression. Silencing might also explain, in part, why genetic twins are non phenotypically identical. In addition, epigenetics is of import for X-chromosome inactivation in female mammals, which is necessary so that females do not have twice the number of 10-chromosome factor products as males (Egger et al., 2004). Thus, the significance of turning genes off via epigenetic changes is readily apparent.
Inside cells, there are 3 systems that can collaborate with each other to silence genes: DNA methylation, histone modifications, and RNA-associated silencing (Figure 1; Egger et al., 2004).
DNA Methylation
Dna methylation is a chemic process that adds a methyl group to Dna. It is highly specific and always happens in a region in which a cytosine nucleotide is located next to a guanine nucleotide that is linked by a phosphate; this is called a CpG site (Egger et al., 2004; Jones & Baylin, 2002; Robertson, 2002). CpG sites are methylated by one of 3 enzymes called DNA methyltransferases (DNMTs) (Egger et al., 2004; Robertson, 2002). Inserting methyl groups changes the appearance and construction of Deoxyribonucleic acid, modifying a gene's interactions with the machinery within a cell's nucleus that is needed for transcription. DNA methylation is used in some genes to differentiate which gene copy is inherited from the father and which gene re-create is inherited from the mother, a phenomenon known as imprinting.
Histone Modifications
Histones are proteins that are the primary components of chromatin, which is the complex of DNA and proteins that makes up chromosomes. Histones deed every bit a spool around which DNA tin can air current. When histones are modified after they are translated into protein (i.e., post-translation modification), they can influence how chromatin is arranged, which, in turn, can determine whether the associated chromosomal Dna will be transcribed. If chromatin is not in a meaty class, it is agile, and the associated DNA can be transcribed. Conversely, if chromatin is condensed (creating a complex called heterochromatin), then it is inactive, and DNA transcription does not occur.
At that place are two main ways histones can be modified: acetylation and methylation. These are chemical processes that add either an acetyl or methyl group, respectively, to the amino acid lysine that is located in the histone. Acetylation is ordinarily associated with agile chromatin, while deacetylation is mostly associated with heterochromatin. On the other hand, histone methylation tin be a marker for both agile and inactive regions of chromatin. For instance, methylation of a particular lysine (K9) on a specific histone (H3) that marks silent Dna is widely distributed throughout heterochromatin. This is the type of epigenetic change that is responsible for the inactivated 10 chromosome of females. In dissimilarity, methylation of a dissimilar lysine (K4) on the same histone (H3) is a marker for active genes (Egger et al., 2004).
RNA-Associated Silencing
Genes can likewise be turned off past RNA when information technology is in the grade of antisense transcripts, noncoding RNAs, or RNA interference. RNA might bear upon gene expression by causing heterochromatin to form, or by triggering histone modifications and Deoxyribonucleic acid methylation (Egger et al., 2004).
Epigenetics and Affliction: Some Examples

While epigenetic changes are required for normal evolution and health, they can besides exist responsible for some disease states. Disrupting any of the three systems that contribute to epigenetic alterations can crusade abnormal activation or silencing of genes. Such disruptions have been associated with cancer, syndromes involving chromosomal instabilities, and mental retardation (Table 1).
Epigenetics and Cancer
The kickoff human illness to exist linked to epigenetics was cancer, in 1983. Researchers found that diseased tissue from patients with colorectal cancer had less Dna methylation than normal tissue from the same patients (Feinberg & Vogelstein, 1983). Because methylated genes are typically turned off, loss of Deoxyribonucleic acid methylation can cause abnormally high factor activation by altering the arrangement of chromatin. On the other paw, too much methylation tin disengage the piece of work of protective tumor suppressor genes.
Every bit previously mentioned, DNA methylation occurs at CpG sites, and a majority of CpG cytosines are methylated in mammals. Notwithstanding, there are stretches of DNA near promoter regions that have college concentrations of CpG sites (known equally CpG islands) that are free of methylation in normal cells. These CpG islands become excessively methylated in cancer cells, thereby causing genes that should not exist silenced to turn off. This aberration is the trademark epigenetic change that occurs in tumors and happens early in the evolution of cancer (Egger et al., 2004; Robertson, 2002; Jones & Baylin, 2002). Hypermethylation of CpG islands can cause tumors by shutting off tumor-suppressor genes. In fact, these types of changes may exist more common in human cancer than Deoxyribonucleic acid sequence mutations (Figure 2).
Furthermore, although epigenetic changes practice not alter the sequence of Dna, they can crusade mutations. Virtually half of the genes that crusade familial or inherited forms of cancer are turned off past methylation. Near of these genes ordinarily suppress tumor germination and help repair DNA, including Ohalf dozen-methylguanine-Dna methyltransferase (MGMT), MLH1 cyclin-dependent kinase inhibitor 2B (CDKN2B), and RASSF1A. For example, hypermethylation of the promoter of MGMT causes the number of Yard-to-A mutations to increase (Figure two).
Hypermethylation can as well lead to instability of microsatellites, which are repeated sequences of Dna. Microsatellites are mutual in normal individuals, and they unremarkably consist of repeats of the dinucleotide CA. Too much methylation of the promoter of the Deoxyribonucleic acid repair gene MLH1 can brand a microsatellite unstable and lengthen or shorten it (Figure 2). Microsatellite instability has been linked to many cancers, including colorectal, endometrial, ovarian, and gastric cancers (Jones & Baylin, 2002).
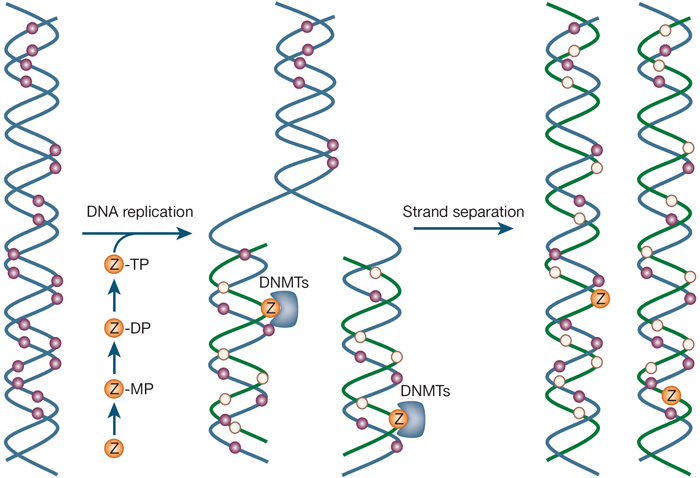
Effigy 2: Mechanism of action of nucleoside analogue inhibitors.
Deoxynucleoside analogues such as 5-aza-ii-deoxycytidine (depicted past Z) are converted into the triphosphate inside S-phase cells and are incorporated in place of cytosine into Dna. Ribonucleosides such as 5-azacytidine or zebularine are reduced at the diphosphate level by ribonucleotide reductase for incorporation (non shown). Once in Dna, the fraudulent bases class covalent bonds with Deoxyribonucleic acid methyltransferases (DNMTs), resulting in the depletion of active enzymes and the demethylation of DNA. Pink circles, methylated CpG; foam circles, unmethylated CpG.
© 2004 Nature Publishing Group Egger, G. et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 460 (2004). All rights reserved. ![]()
Epigenetics and Mental Retardation
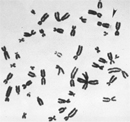
Frail 10 syndrome is the near oft inherited mental disability, specially in males. Both sexes can exist afflicted past this condition, but because males only have one X chromosome, one fragile X will impact them more than severely. Indeed, delicate X syndrome occurs in approximately 1 in 4,000 males and ane in 8,000 females. People with this syndrome accept severe intellectual disabilities, delayed verbal development, and "autistic-similar" behavior (Penagarikano et al., 2007).
Frail X syndrome gets its name from the way the part of the X chromosome that contains the gene abnormality looks under a microscope; it commonly appears as if it is hanging by a thread and hands breakable (Effigy 3). The syndrome is caused by an abnormality in the FMR1 (fragile X mental retardation i) factor. People who exercise not take fragile X syndrome have six to l repeats of the trinucleotide CGG in their FMR1 gene. However, individuals with over 200 repeats have a full mutation, and they commonly prove symptoms of the syndrome. Too many CGGs cause the CpG islands at the promoter region of the FMR1 gene to become methylated; normally, they are non. This methylation turns the factor off, stopping the FMR1 cistron from producing an important protein chosen delicate X mental retardation protein. Loss of this specific protein causes fragile X syndrome. Although a lot of attention has been given to the CGG expansion mutation equally the crusade of fragile X, the epigenetic change associated with FMR1 methylation is the real syndrome culprit.
Frail X syndrome is non the merely disorder associated with mental retardation that involves epigenetic changes. Other such weather include Rubenstein-Taybi, Coffin-Lowry, Prader-Willi, Angelman, Beckwith-Wiedemann, ATR-X, and Rett syndromes (Tabular array 1).
Combating Diseases with Epigenetic Therapy
Because then many diseases, such as cancer, involve epigenetic changes, it seems reasonable to effort to counteract these modifications with epigenetic treatments. These changes seem an ideal target considering they are by nature reversible, unlike DNA sequence mutations. The most popular of these treatments aim to modify either Dna methylation or histone acetylation.
Inhibitors of Deoxyribonucleic acid methylation can reactivate genes that have been silenced. 2 examples of these types of drugs are 5-azacytidine and 5-aza-ii′-deoxycytidine (Egger et al., 2004). These medications work by interim like the nucleotide cytosine and incorporating themselves into DNA while it is replicating. Later they are incorporated into DNA, the drugs block DNMT enzymes from acting, which inhibits DNA methylation.
Drugs aimed at histone modifications are called histone deacetylase (HDAC) inhibitors. HDACs are enzymes that remove the acetyl groups from Deoxyribonucleic acid, which condenses chromatin and stops transcription. Blocking this process with HDAC inhibitors turns on factor expression. The most mutual HDAC inhibitors include phenylbutyric acid, SAHA, depsipeptide, and valproic acid (Egger et al., 2004).
Caution in using epigenetic therapy is necessary considering epigenetic processes and changes are so widespread. To exist successful, epigenetic treatments must be selective to irregular cells; otherwise, activating gene transcription in normal cells could make them cancerous, so the treatments could cause the very disorders they are trying to annul. Despite this possible drawback, researchers are finding ways to specifically target abnormal cells with minimal damage to normal cells, and epigenetic therapy is beginning to look increasingly promising.
References and Recommended Reading
Egger, G., et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463 (2004) doi:10.1038/nature02625 (link to article)
Feinberg, A.P., & Vogelstein, B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92 (1983) doi:10.1038/301089a0 (link to article)
Jones, P. A., & Baylin, Due south. B. The key role of epigenetic events in cancer. Nature Reviews Genetics 3, 415–428 (2002) doi:10.1038/nrg816 (link to article)
Kaati, G., et al. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth flow. European Periodical of Human Genetics 10, 682–688 (2002)
Penagarikano, O., et al. The pathophysiology of fragile 10 syndrome. Annual Review of Genomics and Human Genetics 8, 109–129 (2007) doi:ten.1146/annurev.genom.8.080706.092249
Robertson, K. D. DNA methylation and chromatin: Unraveling the tangled web. Oncogene 21, 5361–5379 (2002) doi:10.1038/sj.onc.1205609
Source: http://www.nature.com/scitable/topicpage/epigenetic-influences-and-disease-895
Posted by: lippertagetaing.blogspot.com


0 Response to "How Does An Epigenetic Change Differ From A Mutation"
Post a Comment